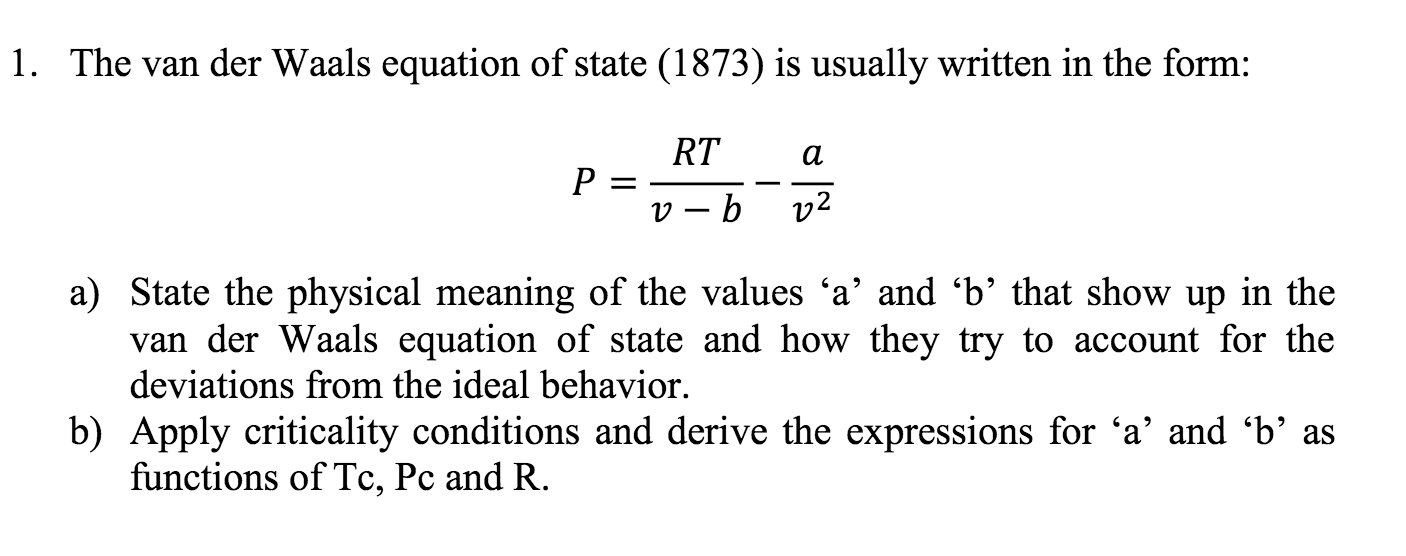
What Does A And B Stand For In Van Der Waals . the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. Check out a few examples. They have positive values and are characteristic of the individual gas. what is van der waals equation of state. What are the constant terms a and b, and their units. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. Learn its derivation, merits, and demerits. van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. the constants a and b are called van der waals constants.
from mungfali.com
van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. the constants a and b are called van der waals constants. what is van der waals equation of state. Check out a few examples. the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: What are the constant terms a and b, and their units. They have positive values and are characteristic of the individual gas. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. Learn its derivation, merits, and demerits. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b.
Van Der Waals Equation
What Does A And B Stand For In Van Der Waals They have positive values and are characteristic of the individual gas. what is van der waals equation of state. Check out a few examples. They have positive values and are characteristic of the individual gas. Learn its derivation, merits, and demerits. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. What are the constant terms a and b, and their units. the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. the constants a and b are called van der waals constants. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b.
From www.aquaportail.com
Force de van der Waals définition et explications What Does A And B Stand For In Van Der Waals Check out a few examples. van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. What are the constant terms a and b, and their units. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and. What Does A And B Stand For In Van Der Waals.
From www.youtube.com
Equação de van der Waals e Princípio dos estados correspondentes YouTube What Does A And B Stand For In Van Der Waals the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. the constants a and b are called van der waals constants. They have positive values and are characteristic. What Does A And B Stand For In Van Der Waals.
From www.chegg.com
Solved Use the van der Waals equation and the constants for What Does A And B Stand For In Van Der Waals van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. What are the constant terms a and b, and their units. Check out a few examples. They have positive values and are characteristic of the individual gas. the constants a and b are. What Does A And B Stand For In Van Der Waals.
From mungfali.com
Van Der Waals Equation What Does A And B Stand For In Van Der Waals Check out a few examples. What are the constant terms a and b, and their units. They have positive values and are characteristic of the individual gas. the constants a and b are called van der waals constants. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b.. What Does A And B Stand For In Van Der Waals.
From www.youtube.com
Van der Waals forces Types and Definitions, formula chemical bonding What Does A And B Stand For In Van Der Waals what is van der waals equation of state. the constants a and b are called van der waals constants. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. Check out a few examples. Learn its derivation, merits, and demerits. the van der. What Does A And B Stand For In Van Der Waals.
From www.electricity-magnetism.org
Ecuación de Van der Waals Guía Simple What Does A And B Stand For In Van Der Waals the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: Learn its derivation, merits, and demerits. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. What are the constant terms a and b, and their units. the constants a and. What Does A And B Stand For In Van Der Waals.
From ecosistemas.win
Cómo calcular volumen con Van der Waals What Does A And B Stand For In Van Der Waals Check out a few examples. the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: what is van der waals equation of state. the constants a and b are called van der waals constants. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply. What Does A And B Stand For In Van Der Waals.
From www.toppr.com
The van der Waals equation for 1 mole of gas is (p + aV^2 )(V b) = RT What Does A And B Stand For In Van Der Waals Check out a few examples. What are the constant terms a and b, and their units. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. what is van der waals equation of state. the correction we’ve introduced is a quantity called the molar volume of gas. What Does A And B Stand For In Van Der Waals.
From hannadesnholson.blogspot.com
Van Der Waals Equation What Does A And B Stand For In Van Der Waals the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: what is van der waals equation of state. the constants a and b are called van der waals constants. van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume. What Does A And B Stand For In Van Der Waals.
From www.youtube.com
Van Der Waal's Constant "a" and "b" Van Der Waal's Equation PART 3 What Does A And B Stand For In Van Der Waals the constants a and b are called van der waals constants. what is van der waals equation of state. Check out a few examples. the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply. What Does A And B Stand For In Van Der Waals.
From brainly.in
Dimensions of a and b in van der waals equation Brainly.in What Does A And B Stand For In Van Der Waals the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: Check out a few examples. what is van der waals equation of state. Learn its derivation, merits, and demerits. They have positive values and are characteristic of the individual gas. the correction we’ve introduced is a quantity called the molar volume. What Does A And B Stand For In Van Der Waals.
From www.youtube.com
How Van der Waals forces work YouTube What Does A And B Stand For In Van Der Waals what is van der waals equation of state. Check out a few examples. Learn its derivation, merits, and demerits. What are the constant terms a and b, and their units. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. the constants a and b are called. What Does A And B Stand For In Van Der Waals.
From www.coursehero.com
[Solved] The van der Waals equation of state was designed (by Dutch What Does A And B Stand For In Van Der Waals They have positive values and are characteristic of the individual gas. the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. Learn its derivation, merits,. What Does A And B Stand For In Van Der Waals.
From scienceinfo.com
Van der Waals Equation Derivation, Correction Factor, Significance What Does A And B Stand For In Van Der Waals the constants a and b are called van der waals constants. Learn its derivation, merits, and demerits. They have positive values and are characteristic of the individual gas. Check out a few examples. What are the constant terms a and b, and their units. the van der waals equation corrects for the volume of, and attractive forces between,. What Does A And B Stand For In Van Der Waals.
From www.expii.com
Van der Waals Forces — Definition & Overview Expii What Does A And B Stand For In Van Der Waals van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. the correction we’ve introduced is a quantity called the molar volume of gas molecules, or simply the van der waals b constant, after. the van der waals equation corrects for the volume. What Does A And B Stand For In Van Der Waals.
From edurev.in
Van der waals, equation of state is (P + a/V2)(V b)= nRT. The What Does A And B Stand For In Van Der Waals the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. the constants a and b are called van der waals constants. . What Does A And B Stand For In Van Der Waals.
From study.com
How to Use the Van der Waals Equation Chemistry What Does A And B Stand For In Van Der Waals the constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b. the van der waals equation corrects for the volume of, and attractive forces between, gas molecules: Check out a few examples. Learn its derivation, merits, and demerits. They have positive values and are characteristic of the individual gas.. What Does A And B Stand For In Van Der Waals.
From eireannmaaya.blogspot.com
13+ Van Der Waals Calculator EireannMaaya What Does A And B Stand For In Van Der Waals what is van der waals equation of state. van der waals constant a and b are related to the intermolecular attractive forces between the gas molecules, and the volume of the molecules respectively. What are the constant terms a and b, and their units. Check out a few examples. the constants a and b are called van. What Does A And B Stand For In Van Der Waals.